UK becomes first to approve Casgevy genetic therapy for blood disorders
SOURCE: HTTPS://INTERESTINGENGINEERING.COM/
NOV 17, 2023
A research partnership brings gene editing promise to eye disease
SOURCE: MORGRIDGE.ORG
AUG 26, 2022
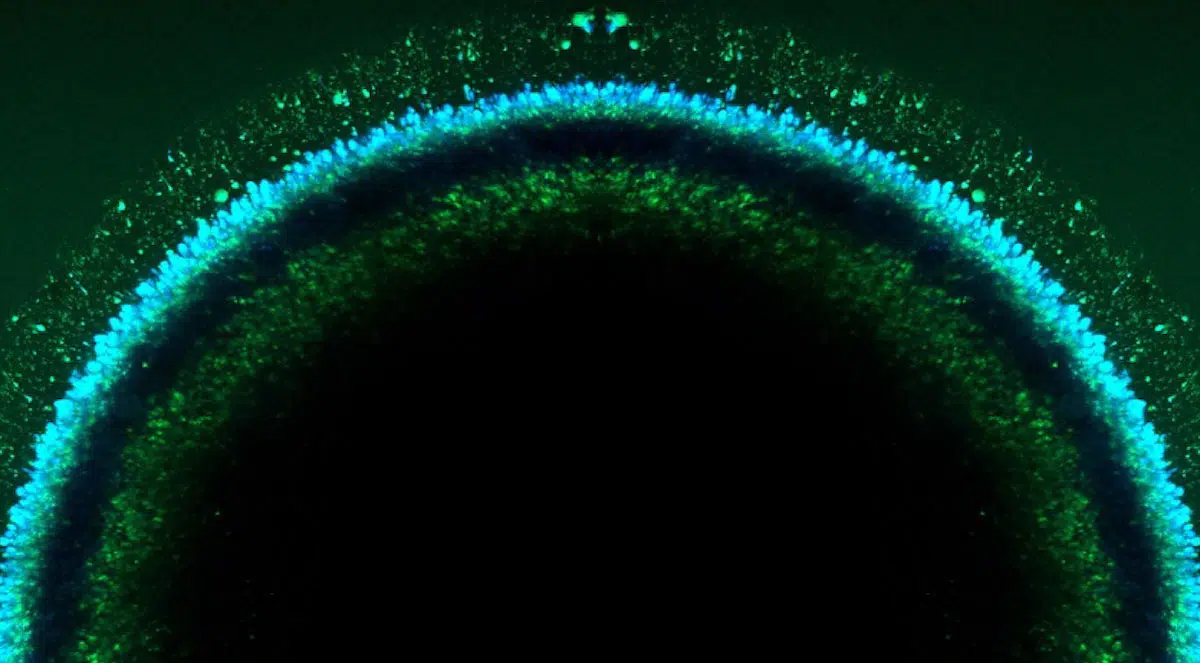
In order to tap the tremendous potential of CRISPR gene editing technology for reversing human disease, Wisconsin scientists are working with a star pupil.
The human eye — though one of the body’s most complex and intricate structures — happens to be an ideal early candidate for treatments that incorporate CRISPR, the Nobel Prize-winning tool that may help precisely target and alter sequences of DNA associated with disease.

David Gamm
David Gamm, director of UW-Madison’s McPherson Eye Research Institute (McPherson ERI) and professor of ophthalmology and visual sciences, describes a number of important attributes. Eyes are a self-contained, compact system. They are readily accessible to treatments, unlike the brain or other organs. They are somewhat immune privileged, making them less prone to tissue rejection. We have two of them — in case something goes wrong.
And there’s major clinical momentum, Gamm says. A gene therapy called Luxturna — used to treat a rare inherited form of vision loss — recently became the first gene therapy approved in the U.S. that targets a disease caused by mutations in a specific gene.
“There’s good reason why the eye is kind of a canary in the coal mine for most of these gene and cell-based therapies,” Gamm says. “Even scientists and companies who are not specifically focused on vision research have recognized that the eye is a great place to start.”
That interest has coalesced at UW-Madison through an interdisciplinary partnership involving scientists at the McPherson ERI, the Morgridge Institute for Research and the Wisconsin Institute for Discovery (WID). The team is organized around a variety of techniques to identify the safety, reproducibility and efficacy of gene editing targets in the eye.
Melissa Skala
Gamm, whose expertise is in cell-based therapies to fight diseases of the retina, started exploring the idea of CRISPR-based therapies about a decade ago with Kris Saha, a biomedical engineer at WID and member of the NIH’s Somatic Cell Genome Editing Consortium. Saha has pioneered new gene editing technologies. They were joined by Morgridge biomedical imaging investigator Melissa Skala, who provides non-invasive ways to measure off-target effects of gene editing. The team also includes Sushmita Roy at WID, who is an expert in computational methods for genome network analysis.
Their NIH-supported project, now in its fourth year, uses stem cell-derived retinal organoids (or tiny 3-D retinal tissue cultures) created in the Gamm Lab to replicate the cellular makeup of eye diseases in a dish. Gamm notes there are more than 250 different genetic diseases that cause human blindness, so creating organoids en masse allows the team to test literally thousands of gene editing combinations to identify beneficial effects and unforeseen safety issues.
“The vast majority of human eye diseases do not have animal models,” Gamm says. “In the case of gene editing, you need to work within the human genome to know if your therapy will be safe and on-target.”
The Skala Lab contributed a technique called autofluorescence lifetime imaging — which can track the natural fluorescence produced during cellular activity. The technology is highly sensitive to retinoids, which are pigments in the eye that change their conformation during visual cycles.

Kayvan Samimi
The experimental approach designed by Skala Lab assistant scientist Kayvan Samimi proved to be effective for tracking the dynamics of cellular changes across these organoids. Importantly, it provides a way to confirm whether changes are occurring as a direct result of the gene editing. Right now, getting that validating proof is one of the major challenges of CRISPR technology.
“It came out of left field,” Skala says. “We all knew that retinoids were in the eye and that they fluoresce, but we thought they weren’t useful to identify function. Kayvan discovered how he could image different conformations of retinoids that were meaningful in determining the function of the cell. So, that was a really fun journey.”
The next step in the journey, Saha says, is to build on the current work to actually develop an investigational new drug based on gene editing of the retina within a patient, rather than treating cells outside the body and returning them. It would represent a monumental step forward, not only in preventing eye diseases but providing gene-editing proof of concept for other diseases. The team submitted an NIH proposal in summer 2022 to take the next step.
“This is a great example of team science and working across colleges and disciplines,” Saha says. “I could tell that we were pushing the boundaries when we first sketched out the project. We were constantly asking: ‘What did you mean by that? What is that term?’ We had to be very specific with our language, we had to slow down. That’s a signal that we’re really bringing together people that normally don’t talk to one another.”
“It’s important to me that I progress my own research mission, and that’s how I choose who I collaborate with,” adds Skala. “If there’s an imaging challenge, then I’m really interested. And in this case, it turned out beautifully because I didn’t even expect this to work out this way.”
Gamm says the project reflects how the McPherson Eye Research Institute approaches basic research. It draws on talented scientists from across the university — many of who have not worked within the eye before — out of the recognition that one field of research won’t have all the answers.
“We’re tapping into what’s already the greatest resource that the UW-Madison has, which is that all of its talented people actually like to work with one another,” Gamm says. “Researchers here are excited to get outside of their own swim lane, and working together is like having a noodle to hang onto as you venture into the deep end.”
LATEST NEWS
Augmented Reality
Hi-tech smart glasses connecting rural and remote aged care residents to clinicians
NOV 20, 2023
WHAT'S TRENDING


Data Science
5 Imaginative Data Science Projects That Can Make Your Portfolio Stand Out
OCT 05, 2022

SOURCE: HTTPS://INTERESTINGENGINEERING.COM/
NOV 17, 2023
SOURCE: HTTPS://GENETICLITERACYPROJECT.ORG/
SEP 05, 2023
SOURCE: HTTPS://WWW.SCIENCEDAILY.COM/
AUG 07, 2023
SOURCE: HTTPS://WWW.SCIENCEDAILY.COM/
JUL 24, 2023
SOURCE: HTTPS://NEWS.MIT.EDU
JUL 20, 2023
SOURCE: BIOSPACE.COM
OCT 27, 2022